A covalent bond is a type of chemical bond which is formed by the mutual sharing of electrons between combining atoms of the same or different elements. According to the orbital concept, a covalent bond is formed between two atoms when a half-filled valence orbital of one atom overlaps with a half-filled valence orbital of the other atom.
Table of Contents
ToggleIn 1916 G. N. Lewis, an American Chemist, suggested that there are atoms which can combine with each other or with other atoms by sharing the unpaired electrons in their outermost shells. In this way the occupied orbitals of the outermost shell of each of the participating atoms are filled with two electrons which have opposite spins. Consequently the paired electrons are shared by both the atoms and circulate about the nuclei of both the atoms. The attractive force of the two nuclei for the shared pair of electrons holds the atoms together and gives rise to the formation of a bond which is called covalent bond or electron pair bond. The compounds containing covalent bonds are called covalent compounds. Each of the two combining atoms contributes the shared electron pair.
According to the Lewis concept when two atoms form a covalent bond, each of the atoms attains the stable configuration of the nearest noble gas, by completing its octet (i.e., 8 electrons in the outermost shell) or doublet (i.e., 2 electrons in case of hydrogen). However, there are many covalent molecules in which the central atom which is covalently bonded with other atoms has electrons either less than eight (incomplete octet) or more than eight (expansion of octet) in its outermost shell. In this way we come across many molecules which have non-octet structure, e.g., BeCl2, BC13, BF3, PCl5, IF7, NO etc. Covalent bonds are formed between the same or different kinds of atoms which should have high electronegativity, since the elements with high electronegativity do not ionize and have an equal attraction for electrons to complete their octets. Such elements are mostly located in right hand portion of the periodic table.
Types of Covalent Bonds
On the basis of electron pair shared between two atoms, there are three types of covalent bond.
1. Single covalent bond
It is the covalent bond which is formed by the sharing of one electron pair between the participating atoms. For example, H₂ molecule is composed of two hydrogen atoms, each having one valence electrons. Each contributes an electron to the shared pair and both atoms acquire helium configuration. Thus stable H₂ molecule results. In F₂ molecule each F-atom (2, 7) has seven valence electrons. The two F atoms acquire a stable electron octet by sharing a pair of electrons.
2. Double covalent bond
If more than one electron pairs are shared between two atoms, then multiple bonds are formed.
When two electron pairs are shared by two atoms, two lines are drawn in the Lewis structure, representing a double bond.
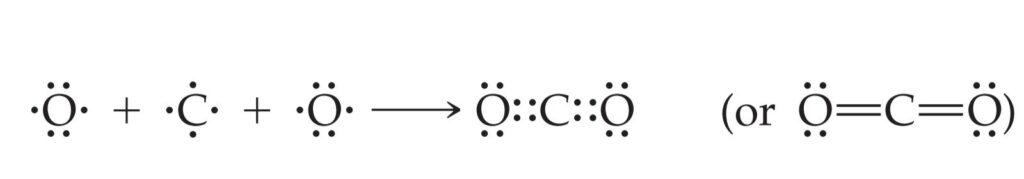
In carbon dioxide, for example, bonding occurs between carbon, with four valence electrons, and oxygen, with six valence electrons.
3. Triple covalent bond
A triple bond corresponds to the sharing of three pairs of electrons, such as in the N2 molecule:
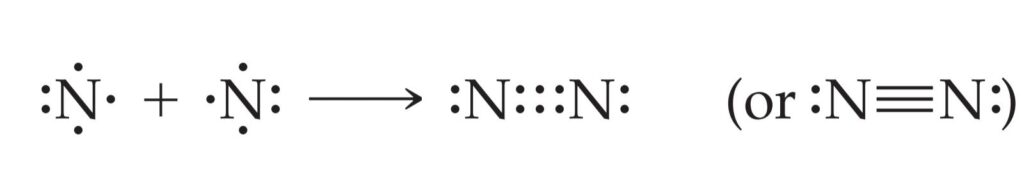
Because each nitrogen atom has five valence electrons, three electron pairs must be shared to achieve the octet configuration.
Covalent Bond Polarity and electronegativity
Bond polarity is a measure of how equally or unequally the electrons in any covalent bond are shared. A nonpolar covalent bond is one in which the electrons are shared equally, as in Cl2 and N2. In a polar covalent bond, one of the atoms exerts a greater attraction for the bonding electrons than the other. If the difference in relative ability to attract electrons is large enough, an ionic bond is formed.
We can use the difference in electronegativity between two atoms to gauge the polarity of the bond the atoms form. Consider these three fluorine-containing compounds:

In F2, the electrons are shared equally between the fluorine atoms and, thus, the covalent bond is nonpolar. A nonpolar covalent bond results when the electronegativities of the bonded atoms are equal.
In HF the fluorine atom has a greater electronegativity than the hydrogen atom, with the result that the electrons are shared unequally—the bond is polar. In general, a polar covalent bond results when the atoms differ in electronegativity. In HF the more electronegative fluorine atom attracts electron density away from the less electronegative hydrogen atom, leaving a partial positive charge on the hydrogen atom and a partial negative charge on the fluorine atom.
In LiF the electronegativity difference is very large, meaning that the electron density is shifted far toward F. The resulting bond is therefore most accurately described as ionic. Thus, if we considered the bond in LiF to be fully ionic, we could say d+ for Li is 1+ and d- for F is 1-. If two atoms differ in electronegativity by more than 2.0, many chemists would consider their bond to be an ionic bond.
There is no sharp distinction between a polar bond and an ionic bond, but the following general rules are helpful as a rough guide.
- An ionic bond forms when the electronegativity difference between the two bonding atoms is 2.0 or more. This rule applies to most but not all ionic compounds.
- A polar covalent bond forms when the electronegativity difference between the atoms is in the range of 0.3 to 2.0.
- A nonpolar covalent bond forms when the electronegativity difference is below 0.3.
Comparison of Properties of ionic and covalent compounds
Ionic and covalent compounds differ markedly in their general physical properties because of differences in the nature of their bonds. There are two types of attractive forces in covalent compounds. The first type is the force that holds the atoms together in a molecule. The second type of attractive force operates between molecules and is called an intermolecular force. Because intermolecular forces are usually quite weak compared with the forces holding atoms together within a molecule, molecules of a covalent compound are not held together tightly. Consequently covalent compounds are usually gases, liquids, or low-melting solids. Further, if intermolecular forces are weak, it is relatively easy to break up aggregates of molecules to form liquids from solids (corresponding to low melting points) and gases from liquids (corresponding to low boiling points).
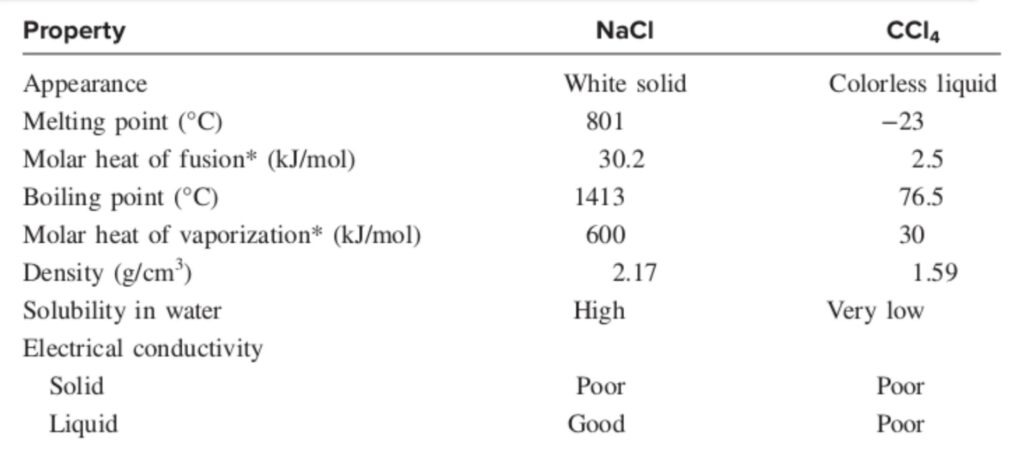
On the other hand, the electrostatic forces holding ions together in an ionic compound are usually very strong, so ionic compounds are solids at room temperature and have high melting points. Many ionic compounds are soluble in water, and the resulting aqueous solutions conduct electricity, because the compounds are strong electrolytes. Most covalent compounds are insoluble in water, or if they do dissolve, their aqueous solutions generally do not conduct electricity because the compounds are nonelectrolytes. Molten ionic compounds conduct electricity because they contain mobile cations and anions; liquid or molten covalent compounds do not conduct electricity because no ions are present. Table given below compares some of the general properties of a typical ionic compound, sodium chloride, with those of a covalent compound, carbon tetrachloride (CCl4).
Covalency;
The valency of an element in a covalent compound is called its covalency. Covalency of an element in a covalent compound is equal to the number of electrons contributed by one atom of it in the shared electron pairs. Consequently, the covalency of an element in a covalent compound is equal to the number of covalent bonds formed by one atom of it with the neighboring atoms. Thus:
Covalency of Cl in Cl2 molecule = 1
Covalency of H in H2, H2O and HCl molecules = 1
Covalency of O in O2 and H2O molecules = 2
Covalency of N in N₂ molecule = 3
Covalency of C in CH4 molecule = 4
Generally the covalency of an element which has only s and p orbitals in its valence- shell (e.g., H, N, O, F) is a fixed quantity and is equal to the number of unpaired electrons in s or p orbitals. However, the elements containing vacant d-orbitals (e.g., P, Cl, S etc.) show different values of covalency in different covalent compounds. This phenomenon is called variable valency and is due to the presence of vacant d-orbitals in the valence-shell of these elements. Variable covalency of an element arises due to the increase in the number of unpaired electrons in the different excited states of the atom.
General characteristics of Covalent compounds
1.Physical state.
Covalent compounds usually consist of discrete molecules and the force of attraction between adjacent covalent molecules is weak. It is due to these weak forces that most of the covalent compounds exist as gases or liquids or soft solids under the normal conditions of temperature and pressure.
2.Melting and boiling points.
With the exception of covalent solids consisting of giant molecules (e.g., diamond, SiC, AIN etc.), other covalent compounds have relatively low melting and boiling points than the ionic compounds. The covalent molecules are held together in the solid crystal lattice by weak forces.
3. Conductance.
Covalent solids consisting of giant molecules are bad conductors of electricity, since they do not contain charged particles or electrons to carry the current. However, the covalent solids having layer lattices (e.g., graphite) are good conductors of electricity, since in such solids electrons can pass from one layer to the other and thus current can be carried.
4. Solubility.
Generally covalent solids are insoluble in polar solvents like H₂O but are readily soluble in non-polar solvents like CCl4, C6H6 etc. Their solubility in non-polar solvents is due to the similarity in covalent nature of the molecules of the solute and solvent, i.e., the solubility is based on the principle: “like dissolves like”. Some of the covalent compounds like alcohol; amines etc. are soluble in water due to hydrogen bonding.
5.Molecular reactions.
The covalent compounds give reactions where the molecule as a whole undergoes a change. Since there are no strong electrical forces to speed up the reaction between molecules, these reactions are slow than ionic reactions.
6. Isomerism.
Since covalent bonds are rigid and directional, they can give rise to different arrangements of atoms in space. This means that covalent compounds can show isomerism and this phenomenon is mostly shown by organic compounds.
7. Neither hard nor brittle.
While ionic compounds are hard and brittle, covalent compounds are neither hard nor brittle. Rather they are soft and waxy, since they usually consist of separate molecules. There are weak forces holding the molecules in the solid crystal lattice.
8. Directional character.
Whereas the ionic bonds are non-directional, the covalent bonds are directional in character. Hence individual covalent compounds possess definite shapes of their molecules.