Chemistry Class 9th
Chapter 01
Fundamentals Of Chemistry
Q No. 2:
What do you understand by the terms mole and Avogadro’s number? Explain with suitable examples.
Table of Contents
ToggleMole
Definition
“The amount of a substance that contains 6.023 × 10²³ particles is known as mole”.
OR
“When the atomic mass, molecular mass, or formula mass are expressed in grams, it is called mole”.
Explanation
Mole is a counting unit used to calculate the amount of a substance. One mole of any substance contains 6.023 × 10²³ particles. The number 6.023 × 10²³ is called Avogadro’s number, named after an Italian scientist Amedo Avogadro who determined this number. Avogadro’s number is represented by NA. One mole of any substance contains 6.023 × 10²³ particles, the term “particles” refers to atoms, molecules, formula units, electrons, etc.
Examples
One mole of H-atoms = 6.023 × 10²³ atoms
One mole of H2O = 6.023 × 10²³ molecules
One mole of NaCl = 6.023 × 10²³ formula units
One mole of Na+ = 6.023 × 10²³ ions
Mole and Molar Mass
Molar mass is the mass of a substance expressed in grams. This mass may be the atomic mass, molecular mass or formula mass of that substance.
Following are the different terms used when the mass of a substance is expressed in grams.
A) Gram atomic mass
When the relative atomic mass of an atom is expressed in grams, instead of amu, it is called gram atomic mass. Gram atomic mass is also known as gram atom. Gram atomic mass is equal to the one mole of that atom. Hence, it is also called 1 mole of that atom.
Examples
- Relative atomic mass of H-atom = 1.008 amu
Gram atomic mass of hydrogen atom = 1.008 g = 1 mol
- Relative atomic mass of oxygen = 15.998 amu
Gram atomic mass of oxygen = 15.998 g = 1 mol
B) Gram molecular mass
When the molecular mass of a molecule is expressed in grams, it is called gram molecular mass. Gram molecular mass is also known as gram molecule. Gram molecular mass is equal to 1 mole of that molecule.
Examples
Gram molecular mass of water = 18g = 1 mol
Gram molecular mass of CO2 = 44g = 1 mol
Gram molecular mass of H2SO4 = 98g = 1 mol
C) Gram formula mass
When the formula mass of an ionic compound is expressed in grams, it is called Gram formula mass. Gram formula mass also known as Gram formula. Gram formula mass is equal to 1 mole of that formula unit.
Examples
Gram formula mass of NaCl = 58.5g = 1 mol
Graham formula mein sab CaCl2 = 111g = 1 mol
Gram formula mass of MgCl2 = 95g = 1 mol
Avogadro’s number
Definition
“The number of particles present in one mole of any substance is called Avogadro’s number”.
Explanation
Avogadro’s number is numerically equal to 6.023 × 10²³ particles. Thus, one mole of any substance contains 6.023 × 10²³ particles. The term “particles” refers to the atoms, molecules, formula units, electrons, etc. Avogadro’s number was determined by an Italian chemist, Amedo Avogadro and this number is represented by NA. Avogadro’s number is a dimensionless quantity. One mole of any substance contains exactly 6.023 × 10²³ particles. If the number of moles are increased, then number of particles present in that substance are also increased and vice versa.
Examples
1g Hydrogen = 1 mole = 6.023 × 10²³ atoms
12g Carbon = 1 mole = 6.023 × 10²³ atoms
18g H2O = 1 mole = 6.023 × 10²³ molecules
58.5g NaCl = 1 mole = 6.023 × 10²³ formula units
Q No. 3:
a) Compare and contrast a mixture and a Give examples of each of them.
b ) How will you classify molecules ? Support your answer with at least two examples of each.
A) Comparing mixture and compounds
Following are the major differences between compound and mixture which are studied to compare and contrast the mixture and compound.
- When different atoms chemically combine (react) with each other, they result in the formation of a compound while mixture is formed by the physically combination (do not react) of two or more substances.
- Compound is a pure form of the substance while mixture is the impure form of the substance.
- The constituents (atoms) of a compound after reacting with each other, loose their original properties while the constituents (substances) of a mixture retain (do not lose) their original properties after mixing because they do not chemically react with each other.
- On the basis of composition, compounds can be classified as organic (carbon containing) compounds and inorganic (other than carbon containing) compounds while mixtures may be classified as homogeneous mixtures or heterogeneous mixtures.
- Compounds have fixed chemical composition while mixtures do not have fixed composition by mass.
- Compounds have uniform composition, so always homogeneous in nature while mixtures can either be homogeneous or heterogeneous.
- The components of the compounds cannot be separated by physical methods due to chemical combination of components but they can be separated by chemical or electrochemical methods while the components of a mixture can be separated by physical methods due to physical combination of the components.
- The properties of a compound are completely different from the properties of constituents while the properties of a mixture are the sum of the properties of its constituents.
- A compound is represented by its chemical formula while mixtures do not have any chemical formula.
- Melting point and boiling point of a compound are always fixed and defined while the melting point and boiling point of a mixture are not fixed and defined.
- Examples of compounds are water, salt, sugar, caustic soda, baking soda, etc., while the examples of mixture are salt in water, sugar in water, air, smog, etc.
B) Classification of molecules
A molecule is formed by the chemical combination of two or more atoms. The smallest unit of a substance is molecule. A molecule may be composed of same or different atoms. Molecules can be classified into different types depending upon the number of combining atoms and nature of combining atoms.
a) On the basis of number of atoms
A molecule may consists of two or more atoms. On the basis of number of atoms, whether same or different, a molecule can be of following types.
- Mono-atomic molecule
Mono means one
A molecule formed by the only one atom is called mono-atomic molecule.
Examples
All noble gases are the examples of monoatomic molecules.
Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe) and Radon (Ra).
- Di-atomic molecule
Di means two
A molecule formed by the chemical combination of only two atoms, whether same or different, is known as di-atomic molecule.
Examples
H2, Cl2, HCl, HBr, O2, N2, etc
- Tri-atomic molecule
Tri means three
A molecule formed by the chemical combination of three atoms, whether same or different, is known as tri-atomic molecule.
Examples
H2O, CO2, O3, NO2, SO2, etc
- Tetra-atomic molecule
Tetra means four
A molecule formed by the chemical combination of four atoms, whether same or different, is known as tetra-atomic molecule.
Examples
NH3 , SO3, P4, etc
- Penta-atomic molecule
Penta means five
A molecule formed by the chemical combination of five atoms, whether same or different, is known as penta-atomic molecule.
Examples
HNO3, CH4, CCl4, CHCl3, CH2Cl2, etc.
- Poly-atomic molecule
Poly means many.
A molecule formed by the chemical combination of many atoms, whether same or different, is known as poly-atomic molecule.
Examples
H2SO4 , C6H6, C6H12O6, S8, etc.
b) On the basis of nature of atoms
A molecule can be made up of same or different atoms. On the basis of nature of combining atoms, a molecule maybe of following two types.
- Homo-atomic molecule
Homo means same.
The type of molecule which is formed by the chemical combination of two or more same atoms is known as homo-atomic molecule.
Examples
H2, O2, N2, P4, S8, Br2, Cl2, etc.
- Hetero-atomic molecule
Hetero means different.
The type of molecule which is formed by the chemical combination of two or more different atoms is known as hetero-atomic molecule.
Examples
HCl, HBr, CO2, H2O, H2SO4, C6H12O6, etc.
Q No. 4:
a) What is the molecular mass of a compound? How will you differentiate it from formula mass.
b) Calculate the molecular mass, or formulae mass, as the case maybe of following compounds in amu.
A) Molecular mass
Definition
“The sum of the relative atomic masses of all the atoms present in a molecule is known as molecular mass of that molecule”.
Explanation
A molecule is composed of two are more atoms. The molecular mass of a molecule is calculated by adding the relative atomic masses of all the atoms present in that molecule. Molecular mass is measured in atomic mass unit (amu). The term “molecular mass” is used more commonly when talking about the mass of a single molecule because different molecules of the same compound may have different molecular masses as they may have different isotopes of the same element.
Examples
- Molecular mass of Ethane
The molecular mass of ethane molecule is calculated by following steps;
Molecular formula of ethane = C2H6
Atomic mass of carbon = 12 amu
Atomic mass of hydrogen = 1 amu
Molecular mass of ethane = 2(C) + 6(H) = 2(12) + 6(1) = 24 + 6 = 30 amu
So, the molecular mass of ethane molecule is 30 amu.
- Molecular mass of Benzene
The molecular mass of benzene can be calculated as ;
Molecular formula of benzene = C6H6
Atomic mass of carbon = 12 amu
Atomic mass of hydrogen = 1 amu
Molecular mass of benzene = 6(C) + 6(H) = 6(12) + 6(1) = 72 + 6 = 78 amu
So, the molecular mass of benzene is 78 amu.
- Molecular mass of Glucose
Molecular formula of glucose = C6H12O6
Atomic mass of carbon = 12 amu
Atomic mass of hydrogen = 1 amu
Atomic mass of oxygen = 16 amu
Molecular mass of glucose = 6(C) + 12(H) + 6(O) = 6(12) + 12(1) + 6(O) = 72 + 12 + 96 = 180 amu
So, the molecular mass of glucose is 180 amu.
Difference between molecular mass and formula mass
Following are the major differences between molecular mass and formula mass.
- Molecular mass is the sum of the atomic masses of all the atoms present in a covalent compound while formula mass is the sum of the masses of all the formula units present in an ionic compound.
- Molecular mass is the term used for the mass of a covalent compound while formula mass is the term used for the mass of an ionic compound.
- Molecular mass is calculated by using molecular formula while formula mass is calculated by using the empirical formula.
- Molecular mass always gives the exact mass of a molecule while formula mass may or may not give the exact mass of a molecule.
- For example, water is a covalent compound, so its mass is the molecular mass which is equal to 18 amu while NaCl is an ionic compound and its mass is the formula mass which is equal to 58.5 amu.
b ) Calculation of Molecular mass and formula mass
- Benzene, C6H6
Benzene is a covalent compound so it will have a molecular mass, which can be calculated as;
Molecular formula of benzene = C6H6
Atomic mass of carbon = 12 amu
Atomic mass of hydrogen = 1 amu
Molecular mass of benzene = 6(C) + 6(H) = 6(12) + 6(1) = 72 + 6 = 78 amu
So, the molecular mass of benzene is 78 amu.
- Ethane gas, C2H6
Ethane is a covalent compound, so it will have a molecular mass which can be calculated as;
Molecular formula of ethane = C2H6
Atomic mass of carbon = 12 amu
Atomic mass of hydrogen = 1 amu
Molecular mass of ethane = 2(C) + 6(H) = 2(12) + 6(1) = 24 + 6 = 30 amu
So, the molecular mass of ethane molecule is 30 amu.
- Aluminium Chloride, AlCl3
Aluminium chloride is a covalent compound, so it will have a molecular mass which can be calculated as;
Molecular formula of aluminium chloride = AlCl3
Atomic mass of Al = 27 amu
Atomic mass of Cl = 35.5 amu
Molecular mass of AlCl3 = 1(Al) + 3(Cl) = 1(27) + 3(35.5) = 133.5 amu
So, the molecular mass of AlCl3 is 133.5 amu.
- Iron oxide, Fe2O3
Iron oxide is an ionic compound, so it will have a formula mass which can be calculated as;
The chemical formula of iron oxide = Fe2O3
Atomic mass of Fe = 56 amu
Atomic mass of O = 16 amu
Formula mass of Fe2O3 = 2(Fe) + 3(O) = 2(56) + 3(16) = 112 + 48 = 160 amu
So, the formula mass of Fe2O3 is 160 amu.
Q No. 5:
a) Find out the number of protons, electrons and neutrons in the following elements.
Na, Ag, Fe, Pb, Ar, U
The number of protons, electrons and neutrons in the following elements can be calculated as;
Na
Atomic number= 11
Atomic mass= 23
And no. Of protons= atomic number
No. Of protons= 11
No. Of electrons= no. Of protons
No. Of electrons= 11
No. Of neutrons= Mass number – no. Of protons
No. Of neutrons= 23 – 11 = 22
Ag
Atomic number= 47
Atomic mass= 107
No. Of protons= atomic number= 47
No. Of electrons= no. Of protons= 47
No. Of neutrons= Mass number – no. Of protons= 107 – 47 = 60
Fe
Atomic number = 26
Mass number = 56
No. Of protons= atomic number= 26
No. Of electrons= no. Of protons= 26
No. Of neutrons= Mass number – no. Of protons= 56 – 26 = 30
Pb
Atomic number= 82
Mass number= 207
No. Of protons= atomic number= 82
No. Of electrons= no. Of protons= 82
No. Of neutrons= Mass number – no. Of protons= 207 – 82 = 125
Ar
Atomic number= 18
Mass number= 40
No. Of protons= atomic number= 18
No. Of electrons= no. Of protons= 18
No. Of neutrons= Mass number – no. Of protons= 40 – 18 = 22
U
Atomic number= 92
Mass number= 238
No. Of protons= atomic number= 92
No. Of electrons= no. Of protons= 92
No. Of neutrons= Mass number – no. Of protons= 238 – 92 = 146
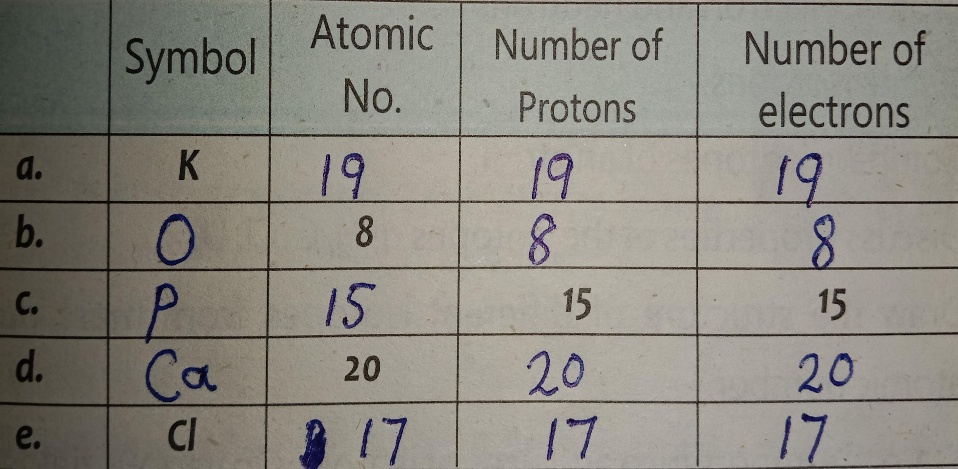
b) Complete the following table.
Read Q NO 1 :- Explain Empirical Formula & Molecular Formula Of A Compound